Radioactive contamination, also called radiological contamination, is the deposition of, or presence of radioactive substances on surfaces or within solids, liquids or gases (including the human body), where their presence is unintended or undesirable (from the International Atomic Energy Agency (IAEA) definition).
Such contamination presents a hazard because of the radioactive decay of the contaminants, which produces such harmful effects as ionizing radiation (namely alpha, beta, and gamma rays) and free neutrons. The degree of hazard is determined by the concentration of the contaminants, the energy of the radiation being emitted, the type of radiation, and the proximity of the contamination to organs of the body. It is important to be clear that the contamination gives rise to the radiation hazard, and the terms "radiation" and "contamination" are not interchangeable.The sources of radioactive pollution can be classified into two
groups: natural and man-made. Following an atmospheric nuclear weapon discharge
or a nuclear reactor containment breach, the air, soil,
people, plants, and animals in the vicinity will become contaminated by nuclear fuel and fission
products.
Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on Saturday 26 April 1986, at the No. 4 reactor in the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian SSR in the Soviet Union. It is considered the worst
nuclear disaster in history both in terms of cost and casualties, and is one of only two nuclear energy accidents rated at seven—the maximum severity—on the International Nuclear Event Scale, the other being the 2011 Fukushima Daiichi nuclear disaster in Japan. The initial emergency response, together with later decontamination of the environment, ultimately involved more than 500,000 personnel and cost an estimated 18 billion Soviet rubles—roughly US$68 billion in 2019, adjusted for inflation.
Although it is difficult
to compare releases and spread of radioactive material between the Chernobyl
accident and a deliberate air burst nuclear detonation, it has still been estimated that
about four hundred times more radioactive material was released from Chernobyl
than by the atomic bombing of Hiroshima and Nagasaki together. At Chernobyl approximately
100,000 square kilometers (39,000 sq mi) of land was significantly
contaminated with fallout, with the worst hit regions being in Belarus, Ukraine
and Russia. Lower levels of contamination were detected over all of Europe
except for the Iberian Peninsula.
Contamination from the Chernobyl accident was scattered irregularly depending on weather conditions, much of it deposited on mountainous regions such as the Alps, the Welsh mountains and the Scottish Highlands, where adiabatic cooling caused radioactive rainfall. The resulting patches of contamination were often highly localized, and localized water-flows contributed to large variations in radioactivity over small areas. Sweden and Norway also received heavy fallout when the contaminated air collided with a cold front, bringing rain. There was also groundwater contamination.
Prior to the completion of the New Safe Confinement building at the reactor No. 4, rainwater acted as a neutron moderator triggering increased fission in the remaining materials risking criticality. Gadolinium nitrate solution was used to quench neutrons to slow the fission.
Even after the completion of the building, fission reactions may be increasing and scientists are working to understand the cause and risks. As of May 2021, while neutron radiation had slowed across most of the destroyed fuel, a sealed off room in the basement had actually recorded a doubling in neutron radiation. This indicated increasing levels of fission as water levels dropped, which was the opposite of what was expected, and was a typical compared to other fuel containing areas. Levels are increasingly slowly, so scientists are expected to have several years to solve the problem. However, if the trend continues it could create a self-sustaining reaction, which would likely spread more radioactive dust and debris through the New Safe Confinement, making future cleanup even more difficult. Potential solutions include using a robot to drill into the fuel and insert boron carbide control rods.
Fukushima
Daiichi nuclear disaster
The Fukushima Daiichi nuclear disaster was a
2011 nuclear accident at
the Fukushima
Daiichi Nuclear Power Plant in Ōkuma, Fukushima Prefecture, Japan.
The event was caused by the 2011 Tōhoku earthquake and tsunami.
It was the most severe nuclear accident since the Chernobyl disaster in
1986. It was classified as Level 7 on the International
Nuclear Event Scale (INES), after initially being classified as
Level 5, joining Chernobyl as the only other accident to receive such
classification.
The accident was triggered by the Tōhoku earthquake and tsunami on Friday, 11 March 2011. On detecting the earthquake, the active reactors automatically shut down their normal power-generating fission reactions. Because of these shutdowns and other electrical grid supply problems, the reactors electricity supply failed, and their emergency diesel generators automatically started. Critically, these were required to provide electrical power to the pumps that circulated coolant through the reactors cores. This continued circulation was vital to remove residual decay heat, which continues to be produced after fission has ceased. However, the earthquake had also generated a tsunami 14 meters (46 ft) high that arrived shortly afterwards and swept over the plant's seawall and then flooded the lower parts of reactors 1–4. This flooding caused the failure of the emergency generators and loss of power to the circulating pumps. The resultant loss of reactor core cooling led to three nuclear meltdowns, three hydrogen explosions, and the release of radioactive contamination in Units 1, 2 and 3 between 12 and 15 March 2011. The spent fuel pool of previously shut down Reactor 4 increased in temperature on 15 March due to decay heat from newly added spent fuel rods, but did not boil down sufficiently to expose the fuel.
In the days after the accident, radiation released to the
atmosphere forced the government to declare an ever-larger evacuation zone
around the plant, culminating in an evacuation zone with a 20 km radius. All
told, some 154,000 residents evacuated from the communities surrounding the
plant due to the rising off-site levels of ambient ionizing radiation caused
by airborne radioactive contamination from the damaged reactors.
Large amounts of water contaminated with radioactive isotopes were
released into the Pacific Ocean during and after the disaster. Michio Aoyama, a professor of radioisotope geoscience at the Institute of Environmental Radioactivity, has estimated that 18,000 terabecquerel (TBq) of radioactive caesium-137 were released into the Pacific during the accident, and in 2013, 30 gigabecquerel (GBq) of caesium 137 were still flowing into the ocean every day. The plant's operator has since built new walls along the coast and has created a 1.5 km long "ice wall" of frozen earth to stop the flow of contaminated water.
In June 2011, TEPCO stated the amount of
contaminated water in the complex had increased due to substantial rainfall. On
13 February 2014, TEPCO reported 37 kBq (1.0 microcurie) of caesium-134 and
93 kBq (2.5 microcuries) of caesium-137 were
detected per liter of groundwater sampled from a monitoring well. Dust
particles gathered 4 km from the reactors in 2017 included microscopic
nodules of melted core samples encased in cesium. After decades of
exponential decline in ocean cesium from weapons testing fallout, radioactive
isotopes of cesium in the Sea of Japan increased
after the accident from 1.5 mBq/L to about 2.5 mBq/L and are still
rising as of 2018, while those just off the eastern coast of Japan are
declining.
Since the 2011 Fukushima Daiichi nuclear disaster, the nuclear plant has accumulated 1.25 million tonnes of waste water, stored in 1,061 tanks on the land of the nuclear plant, as of March 2021. It will run out of land for water tanks by 2022. It has been suggested the government could have solved the problem by allocating more land surrounding the power plant for water tanks, since the surrounding area had been designated as unsuitable for humans. Regardless, the government was reluctant to act. Mainichi Shimbun criticized the government for showing "no sincerity" in "unilaterally push[ing] through with the logic that there will no longer be enough storage space.
On 13 April 2021, the Cabinet of Prime Minister Suga unanimously
approved that TEPCO dump the stored water to the Pacific Ocean over a course of
30 years. The Cabinet asserted the dumped water will be treated and diluted to drinkable standard. The idea of dumping
had been floated by Japanese experts and officials as early as June 2016.
Caesium-137
Caesium-137 (13755Cs), or radiocaesium, is a radioactive isotope of caesium that is formed as one of the more common fission products by the nuclear fission of uranium-235 and
other fissionable isotopes in nuclear reactors and nuclear weapons. Trace quantities also originate from natural fission of uranium-238. It is among the most problematic of the short-to-medium-lifetime fission products. When suddenly released at high temperature, as in the case of the Chernobyl nuclear accident and with atomic bombs explosions, because of the relatively low boiling point (671 °C, 1240 F) of the element, 137Cs is easily volatilized in the atmosphere and transported in the air on very long distances. After the radioactive fallout, it is deposited onto the soil and easily moves and spreads in the environment because of the high water solubility of caesium's most common chemical compounds, which are salts. 137Cs was discovered by Glenn T. Seaborg and Margaret Melhase.
Decay
Caesium-137 has a half-life of about 30.17 years. About
94.6% decays by beta emission to a metastable nuclear isomer of barium: barium-137m (137mBa, Ba-137m). The remainder directly populates the
ground state of barium-137, which is stable. Metastable barium has a half-life
of about 153 seconds, and is responsible for all of the gamma ray emissions in samples of caesium-137. 137mBa decays to the ground state by emission of photons having energy 0.6617 MeV. A
total of 85.1% of 137Cs decays lead to gamma ray emission in this way.
One gram of caesium-137 has an activity of 3.215 terabecquerel (TBq).
Health
risk of radioactive caesium
Caesium-137 reacts with
water, producing a water-soluble compound (caesium hydroxide). The biological behaviour of caesium is similar
to that of potassium and rubidium. After entering the body, caesium gets more or
less uniformly distributed throughout the body, with the highest concentrations
in soft tissue. The biological half-life of caesium is about 70 days.
Important researches have
shown a remarkable concentration of 137Cs in the exocrine cells of the pancreas, which
are those most affected by cancer. In 2003, in autopsies performed
on 6 children dead in the polluted area near Chernobyl where they also reported
a higher incidence of pancreatic tumors, Bandazhevsky found a concentration of 137Cs 40-45 times higher than in their liver, thus
demonstrating that pancreatic tissue is a strong accumulator and secretor in
the intestine of radioactive cesium.
Radioactive caesium in the environment
Caesium-137, along with other radioactive isotopes caesium-134, iodine-131, xenon-133,
and strontium-90,
were released into the environment during nearly all nuclear weapon tests and
some nuclear accidents, most notably the Chernobyl disaster and
the Fukushima Daiichi disaster.
Chernobyl disaster
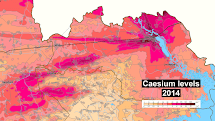
As of today and for the next few hundred years or so, caesium-137 and strontium-90 continue to be the principal source of radiation in the zone of alienation around the Chernobyl nuclear power plant, and pose the greatest risk to health, owing to their approximately 30 year half-life and biological uptake. The mean contamination of caesium-137 in Germany following the Chernobyl disaster was 2000 to 4000 Bq/m2. This corresponds to a contamination of 1 mg/km2 of caesium-137, totaling about 500 grams deposited over all of Germany. In Scandinavia, some reindeer and sheep exceeded the Norwegian legal limit (3000 Bq/kg) 26 years after Chernobyl. As of 2016, the Chernobyl caesium-137 has decayed by half, but could have been locally concentrated by much larger factors.
Fukushima Daiichi disaster
In April 2011, elevated levels of caesium-137 were also being found in the environment after the Fukushima Daiichi nuclear disasters in Japan. In July 2011, meat from 11 cows shipped to Tokyo from Fukushima
Prefecture was found to have 1,530 to 3,200 becquerels per kilogram of 137Cs, considerably exceeding the Japanese legal limit of 500 becquerels per kilogram at that time. In March 2013, a fish caught near the plant had a record 740,000 becquerels per kilogram of radioactive caesium, above the 100 becquerels per kilogram government limit. A 2013 paper in Scientific Reports found that for a forest site 50 km from the stricken plant, 137Cs concentrations were high in leaf litter, fungi and detritivores, but low in herbivores. By the end of 2014, "Fukushima-derived radiocaesium had spread into the whole western North Pacific Ocean", transported by the North Pacific current from Japan to the Gulf of Alaska. It has been measured in the surface layer down to 200 meters and south of the current area down to 400 meters.